Answer:
The temperature of cold reservoir should be 246.818 K for efficiency of 35%
Step-by-step explanation:
In first case we have given efficiency of Carnot engine = 26 % = 0.26
Temperature of cold reservoir
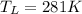
We know that efficiency of Carnot engine is given by
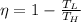
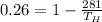
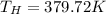
For second Carnot engine efficiency is given as 35% = 0.35
And temperature of hot reservoir is same so
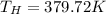
So
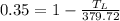
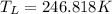
So the temperature of cold reservoir should be 246.818 K for efficiency of 35%