Answer:
Step-by-step explanation:
The density of pure lead is 10.49g/cm³
Given parameters:
Volume of water displaced by the crown = 238.1ml
Mass of crown = 2.50kg
Solution
We would simply find the density of the discovered crown and compare with that of the standardized density of lead.
Density is an intensive property of matter and it is the same for any form of matter. It is amount of substance per unit volume:
Density =
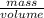
We need to convert the given parameters to standard unit,
1ml = 1cm³
Therefore, the volume of water displaced is 238.1cm³
Also,
1kg = 1000g
2.5kg = 2500g
mass of the crown is 2500g
Density =
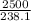 = 10.49g/cm³
= 10.49g/cm³
The crown is made of silver because the density matches with that of pure silver.