Answer:
54.9 mg of the radioisotope are still active after 110 min.
Step-by-step explanation:
Half-life of F-18 is found to be 109.7 minutes
Rate constant
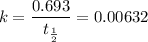
t = time taken = 110 minutes
![$\ln [A]=\ln [A]_(0)-k t$](https://img.qammunity.org/2020/formulas/chemistry/middle-school/fcquyd0kkfrjb4xy6vyyu6ov40f190c2n4.png)
[A] is the final quantity
![[A]_0](https://img.qammunity.org/2020/formulas/chemistry/college/1z3zdal3zhydky3w24hmgrwjngt8hmnwl0.png) is the initial quantity
is the initial quantity
Plugging the values and solving for [A]
![\\$\ln [A]=\ln (110 m g)-\left(0.00632 \min ^(-1) * 110 \min \right)$\\\\$\ln [A]=4.700-0.6952$\\\\$\ln [A]=4.0048$\\\\$[A]=e^(4.0048)$](https://img.qammunity.org/2020/formulas/chemistry/middle-school/dryrz3ufvmb5ik965j13gjhb894yjfenl9.png)
[A] = 54.9 mg is the Answer