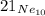Answer:
The symbol of the isotope is
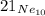
Step-by-step explanation:
Nucleus symbol is have three parts in it, such as- The atomic number of the element, the symbol of the element and the mass number of the element. The form of a unique chemical element which has a certain number of neutron and proton in its atomic nucleus is known as isotope of that element.
An element can have one or more isotopes. Neon has three isotopes, all of which are stable. The symbol of neon is- Ne, the atomic number of neon is- 10 and the isotope of neon which has 11 neutrons is- Ne21. Thus the symbol of that isotope is