Answer:
10 is the total number of electrons present in that atom.
Step-by-step explanation:
The formula for total number of electron in nth shell is given by :
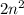
First shell = 1
Number of electron in first shell,n = 1:
=
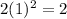
Number of electron in first shell,n = 2:
=
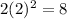
Total number of electrons in the atom with full first shell and second shell:
2 + 8 = 10