Answer:
A reaction is said to occur if there is a formation of an insoluble solid or a precipitate(s) or a liquid (l) or a gas(g).
If both the reactants and products are in aqueous state, No reaction takes place.
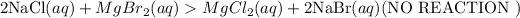
All chlorides and Bromides are soluble except that of Ag, Hg and Pb.
Hence, No reaction takes place since all the reactants and products are in aqueous states.
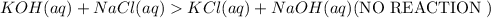
Salts of Group IA are soluble. Hence No reaction takes place
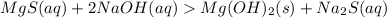
(REACTION TAKES PLACE)
All hydroxides are insoluble except that of Group IA, ammonium ion and Group IIA down from Calcium.
Hence Reaction takes place with the formation of
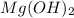 precipitate
precipitate