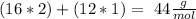Answer:
Step-by-step explanation:
If we want to calculate the molar mass of this compound we have to find the atomic masses of each atom, in this case:
Oxygen =
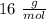
Carbon=
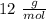
Then we have to multiply the atomic mass by the number of atoms in the compound, so: