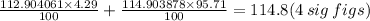Step-by-step explanation:
I'm not sure if this is what you are looking for but i will attempt to answer.
Isotopes are variations of the same atom. They have the same number of protons but have a different number of neutrons. As a result of this, the atomic number remains the same but the mass number changes.
A calculation you could perform in relation to isotopes would be calculating the relative atomic mass. The relative atomic mass is the weighted average of masses of isotopes.
Relative atomic mass (RAM)= the addition of

For example, the element Indium has a relative isotopic mass of 112.90406, 4.29% of the time. It has a relative isotopic mass of 114.903878, 95.71% of the time.
From this
RAM=