Answer:
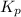 for the reaction is 18.05
for the reaction is 18.05
Step-by-step explanation:
Equilibrium constant in terms of partial pressure (
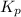 ) for this reaction can be written as-
) for this reaction can be written as-
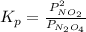
where
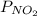 and
and
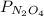 are equilibrium partial pressure of
are equilibrium partial pressure of
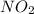 and
and
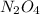 respectively
respectively
Hence
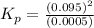 = 18.05
= 18.05
So,
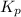 for the reaction is 18.05
for the reaction is 18.05