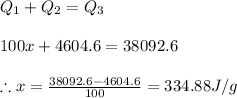Answer:
The latent heat of fusion of water is 334.88 Joules per gram of water.
Step-by-step explanation:
Let the latent heat of ice be 'x' J/g
1) Thus heat absorbed by 100 gram of ice to get converted into water equals
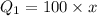
2) heat energy required to raise the temperature of water from 0 to 25 degree Celsius equals
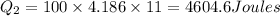
Thus total energy needed equals
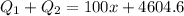
3) Heat energy released by the decrease in the temperature of water from 25 to 11 degree Celsius is
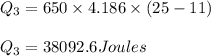
Now by conservation of energy we have