Answer:
45.95 grams of iron
Step-by-step explanation:
The reaction between FeO and magnesium metal is
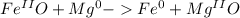
1. The first step is verify that the equation is balanced. In this case it is balanced since the numbers of atoms of each element are equal in both sides of equation (for example we have 1 Fe atom in reactants and 1 Fe atom in products)
2. We need to calculate the limiting reactant
- for that we need to calculate the number of moles of each reactant dividing the mass that we have by the respective molecular weight of the compound.
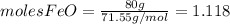 and
and
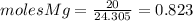 the molecular weight of Mg (24.305) can be readed in the periodic table of elements.
the molecular weight of Mg (24.305) can be readed in the periodic table of elements. - so we divide the moles by stoichiometry number (number in front of each compound in the equation) in this case is 1 for both reactants (that is we need 1 mol of FeO and 1 mol of Mg to produce 1 mol of Fe).
- The lower number obtained was 0.823 for Mg, so Mg is the limiting reactant.
3. We calculate the moles and the mass of Fe produced. The maximum number of moles of Fe that can be produced is given by the limit reactant. So we would use the moles of Mg to calculate the Fe produced.
- We have 0.823 mol of Mg and the chemical equation shown above say that we need 1 mol of Mg to produce 1 mol of Fe, so with 0.823 mol of Mg we woud produce 0.823 mol of Fe (
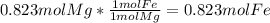 ).
). - To convert from mol of Fe to grams of Fe we would multiply by the molecular weight of Fe
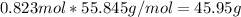 (molecular weight of Fe is readed in the periodic table of elements). So it is produced 45.95 g of iron
(molecular weight of Fe is readed in the periodic table of elements). So it is produced 45.95 g of iron