Answer:
Valance electron in
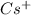 will be zero
will be zero
Step-by-step explanation:
We know that atomic number of cesium is 55
The electronic configuration of Cs
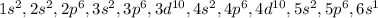
Currently therte is one valance electron in Cs
We have given
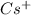 + sign indicates that cesium losses one electron means now it have no valance electron.
+ sign indicates that cesium losses one electron means now it have no valance electron.
So valance electron in
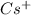 will be zero
will be zero