Answer:
12% mass/volume
Step-by-step explanation:
The mass volume percent concentration (%m/v) expresses how much grams of solute are dissolved in 100 ml of solution, and it can be calculated with the following expression:
% m/v = g solute/100 ml solution
We have 1.8 g solute (heparin sodium) in 15 ml of solution, so we can obtain %m/v as follows:
%m/v=
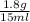 x 100 ml = 12 % m/v
x 100 ml = 12 % m/v