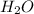Answer:
Ion
Ion is a charged particle.
A neutral atom contains equal number of positive protons and negative electrons
When an electron is lost or gained the atom becomes a charged particle which is called as an ION.
For example
Na is an atom which contains 11 electrons and 11 protons(= to atomic number)
When Na loses an electron it will have 11 positive proton and 10 negative electron and Thus, Na will become
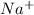 , a charge particle which we call it as sodium ion.
, a charge particle which we call it as sodium ion.
Cl is an atom which contains 17 electrons and 17 protons(= to atomic number)
When Cl loses an electron it will have 17 positive proton and 18 negative electron and Thus, Cl will become
 , a charge particle which we call it as chloride ion.
, a charge particle which we call it as chloride ion.
When an ion is positively charged it is called as a cation
When an ion is negatively charged it is called as an anion.
Salt is formed when an acid reacts with a base.
The cation of the base combines with the anion of the acid to form the salt.
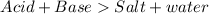
HCl is acid and NaOH is a base forms salt NaCl and water.
 is an anion of the acid and
is an anion of the acid and
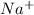 is the cation of the base combines to form NaCl salt .
is the cation of the base combines to form NaCl salt .
 and
and
 combines to form
combines to form