Answer: The voltage of the cell is 1.05 V.
Step-by-step explanation:
The given cell is:
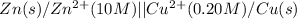
Half reactions for the given cell follows:
Oxidation half reaction:
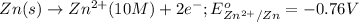
Reduction half reaction:
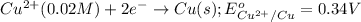
Net reaction:
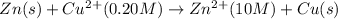
Oxidation reaction occurs at anode and reduction reaction occurs at cathode.
To calculate the
 of the reaction, we use the equation:
of the reaction, we use the equation:
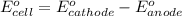
Putting values in above equation, we get:
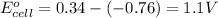
To calculate the EMF of the cell, we use the Nernst equation, which is:
![E_(cell)=E^o_(cell)-(0.059)/(n)\log ([Zn^(2+)])/([Cu^(2+)])](https://img.qammunity.org/2020/formulas/chemistry/college/z8hza8vgjawa024fk9s0qonfqhq53xhor7.png)
where,
 = electrode potential of the cell = ?V
= electrode potential of the cell = ?V
 = standard electrode potential of the cell = +1.1 V
= standard electrode potential of the cell = +1.1 V
n = number of electrons exchanged = 2
![[Cu^(2+)]=0.02M](https://img.qammunity.org/2020/formulas/chemistry/college/7c06qbq0ey4n8xe2s6p1rykypa4fls35hb.png)
![[Zn^(2+)]=10M](https://img.qammunity.org/2020/formulas/chemistry/college/1a1h7k5zl6dx8nlc8ymx9yd4i1sf8k0280.png)
Putting values in above equation, we get:
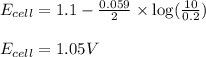
Hence, the voltage of the cell is 1.05 V.