Step-by-step explanation:
Given that,
Wavelength of the photon,
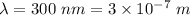
Work function of the metal,
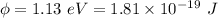
We need to find the maximum kinetic energy of the ejected electrons. It can be calculated using Einstein's photoelectric equation as :

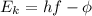
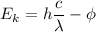
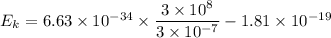
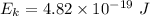
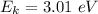
or
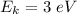
So, the maximum kinetic energy of the ejected electrons is 3 ev. Hence, this is the required solution.