Answer:
B. pI=10
C. Charge 1+
Step-by-step explanation:
B. You'll write your pKa values from low to high, we'll build a table to find the charges for your three posible dissociated groups:
-COOH -NH3 -R Total charge
0 + + 2+
pH 3 -----------------------------------------------------
- + + 1+
pH 9------------------------------------------------------
- 0 + 0
pH 11------------------------------------------------------
- 0 0 1-
To calculate pI we'll take the pKa values where you have total charge zero (pKa 9 and 11):
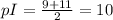
Our pI=10
C. considering pH 6 is between pH3 and pH9 we can assume that the net charge in that pH will be 1+.
I hope you find this information useful! Good luck!