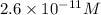Answer : The correct option is, (A)
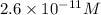 , acidic
, acidic
Step-by-step explanation:
pH : It is defined as the negative logarithm of hydrogen ion or hydronium ion concentration.
When the value of pH is less then 7 then the solution will be acidic.
When the value of pH is more then 7 then the solution will be basic.
When the value of pH is equal to 7 then the solution will be neutral.
First we have to calculate the pH.
![pH=-\log [H^+]](https://img.qammunity.org/2020/formulas/chemistry/high-school/ipfjz05f4cfbguiwup37xvxa7furlbuapf.png)
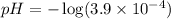
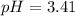
Now we have to calculate the pOH.
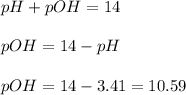
Now we have to calculate the
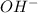 concentration.
concentration.
![pOH=-\log [OH^-]](https://img.qammunity.org/2020/formulas/chemistry/high-school/h1t4ubcsdqvqg0xpalkkvnwrun04y9pzd8.png)
![10.59=-\log [OH^-]](https://img.qammunity.org/2020/formulas/chemistry/college/h09dr9vusx75jg6az86yrzytpjhlt9c0xc.png)
![[OH^-]=2.6* 10^(-11)M](https://img.qammunity.org/2020/formulas/chemistry/college/7u5xp918ko8xg6ycombn0s3vi11tfgwdal.png)
Therefore, the
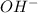 concentration is,
concentration is,