Answer:
2,1,-1
Step-by-step explanation:
The electronic configuration of oxygen is
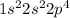
The valence electrons are in the second principle quantum number, which means that the value of n = 2.
For, n =2,
l can be 0 or 1 which corresponds to s and p orbital respectively.
If l = 1 , which is p orbitals and it exits in 3 degenerate orbitals which has values of m = -1 , 0 , +1
So, The possible set pf quantum number for the outermost valence electron of the oxygen atom is 2, 1, -1.