Answer:
Concentration of the first solution: approximately 0.200 mol/L.
Concentration of the diluted solution: approximately 0.0250 mol/L.
Step-by-step explanation:
Refer to a modern periodic table for relative atomic mass data:
- H: 1.008;
- S: 32.06;
- O: 15.999.
Formula mass of sulfuric acid
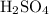 :
:
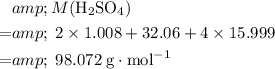 .
.
Number of moles of
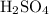 in
in
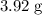 of this substance:
of this substance:
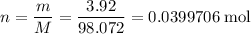 .
.
Convert cubic centimeters to liters:
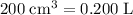 .
.
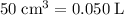 .
.
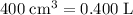 .
.
Concentration of this solution:
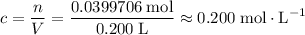 .
.
While both the volume and the concentration of the
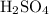 solution changes when it is diluted, the number of moles of
solution changes when it is diluted, the number of moles of
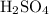 in this solution will stay the same. Number of moles of
in this solution will stay the same. Number of moles of
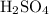 in this
in this
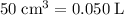 of concentrated solution:
of concentrated solution:
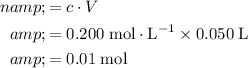 .
.
That will be the same as the number of moles of
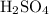 in the diluted solution.
in the diluted solution.
Concentration of this solution after it is diluted to
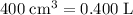 :
:
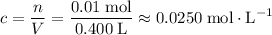 .
.