Answer:
14.9 hrs
Explanation: Let N₀ and N be numbers of atoms in the beginning and after 48 hours of duration respectively. λ be the disintegration constant.
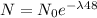
dN₀ / dt = λN₀
dN / dt = λN
Given
dN / dt = .1072 x dN₀ / dt
λN = .1072 x λN₀
N = .1072 x N₀
N₀e^{-\lambda 48 =.1072 x N₀
e^{-\lambda 48 =.1072
Taking ln on both sides
- λ x 48 = ln .1072
λ x 48 = 2.233
λ = 2.233 / 48
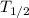 = .693 /λ
= .693 /λ
=( .693 /2.233 )X 48
= 14.9 hrs.