Answer : The pH of buffer is 9.06.
Explanation : Given,
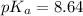
Concentration of HBrO = 0.34 M
Concentration of KBrO = 0.89 M
Now we have to calculate the pH of buffer.
Using Henderson Hesselbach equation :
![pH=pK_a+\log ([Salt])/([Acid])](https://img.qammunity.org/2020/formulas/chemistry/college/6wyuhr9b7n0qwlgrnwgg688yylfbvv3wby.png)
![pH=pK_a+\log ([KBrO])/([HBrO])](https://img.qammunity.org/2020/formulas/chemistry/college/1vpagkwbfjpvhhf3q186et3j0r1huemc69.png)
Now put all the given values in this expression, we get:
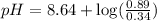
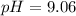
Therefore, the pH of buffer is 9.06.