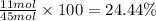Step-by-step explanation:
Molecular mass of sugar =
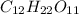 : = 432 g/mol
: = 432 g/mol
Atomic mass of carbon atom = 12 g/mol
Atomic mass of hydrogen atom = 1 g/mol
Atomic mass of oxygen atom = 16 g/mol
a) Percentage of an element in a compound:
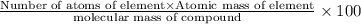
Percentage of carbon by weight in
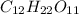 :
:
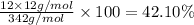
Percentage of hydrogen by weight in
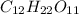 :
:
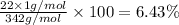
Percentage of oxygen by weight in
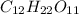 :
:
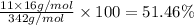
b) Percentage of mole each of the elements present in sugar:
=
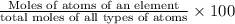
In mole of sugar we have 12 moles of carbon atom , 22 moles of hydrogen atoms and 11 moles of oxygen atoms.
Percentage of carbon by mole in
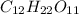 :
:
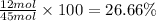
Percentage of hydrogen by mole in
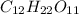 :
:
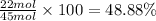
Percentage of oxygen by mole in
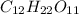 :
: