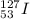Answer:
The atomic symbol of iodine-127 is
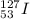
Step-by-step explanation:
Looking iodine up in the periodic table we find it in the
 column and in the fifth period. We find that its atomic symbol is I.
column and in the fifth period. We find that its atomic symbol is I.
In the periodic table we can also find a lot of data about this element like its electronegativity, its relative atomic mass (an average of the number of protons and neutrons in the different isotopes, 126,90 in this case) and its electron configuration, but more important, that the atomic number (number of protons, characteristic that defines an element) of iodine is 53.
This isotope has a mass number of 127, it is the most common isotope found on Earth and the only one that is stable (it doesn't decay into other elements, i.e, it isn't radioactive).
Knowing all this, the atomic symbol of this isotope of iodine is