Answer:
(a)
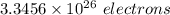
Step-by-step explanation:
Given:
- Volume of water = 1 L
- Mass per mole of water molecule = 18.0 g/mol
- Number of electrons per molecule of water = 10 electron/mole
Assume:
- Density of water = 1 kg/L
Since the density of water is 1 kg/L. This means 1 L of water has a mass of 1 kg.
Mass of 1 L water = 1 kg = 1000 g
It is given that 1 mole of water molecule weighs 18.0 g. From this value, we can find the number of moles of water molecules in 1000 g water as below:
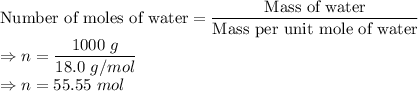
Since, 1 mole of any substance contains
 atoms.
atoms.
Let us calculate the total number of molecules of water which is given by:
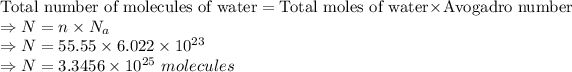
Since one molecule of water contains 10 electrons. So, the given number of molecules of water contains the following electrons which is as:
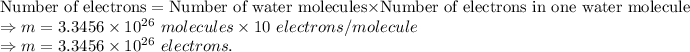
Hence, 1 liter of water contains
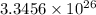 electrons.
electrons.