Answer:
539.19 m/s , 459.83 m/s , 2156.78 m/s
Step-by-step explanation:
The formula for the rms speed is given by
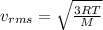
Where, R be the gas constant, T be the absolute temperature and M be the molecular mass of the gas
R = 8.314 in SI units
T = 100°C = 100 + 273 = 373 K
For Oxygen:
M = 32 g = 0.032 kg
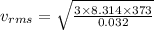
vrms = 539.19 m/s
For carbon di oxide:
M = 44 g = 0.044 kg
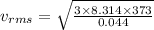
vrms = 459.83 m/s
For hydrogen:
M = 2 g = 0.002 kg
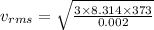
vrms = 2156.78 m/s