Answer:
(a) 13.6 eV
(b) 10.2 V
Step-by-step explanation:
a) Ionization potential energy is the least requirement of energy to fetch a neutral atom from its initial state to its ionized state i.e basically the minimum energy required to excite an electron from n=1 to infinity.
Energy of a level, n, in Hydrogen atom is,
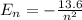
Now ionization potential can be calculated as

Substitute all the value of energy and n in above equation.
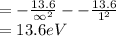
Therefore, the ionization potential is 13.6 eV.
b) This is the energy required to excite a atom from ground state to its excited state. When electrons jumps from ground state level(n=1) to 1st excited state(n=2) the corresponding energy is called 1st excitation potential energy and corresponding potential is called 1st excitation potential.
So, 1st excitation energy = E(n 2)- E(n = 1)
So,
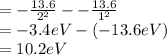
Now we can find that 1st excitation energy is 10.2 eV which gives,
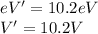
Therefore, the 1st excitation potential is 10.2V.