Answer:
a) 23.2 e V
b) energy of the original photon is 36.8 eV
Step-by-step explanation:
given,
energy at ground level = -13.6 e V
energy at first exited state = - 3.4 e V
A photon of energy ionized from ground state and electron of energy K is released.
h ν₁ - 13.6 = K
K combine with photon in first exited state giving out photon of energy
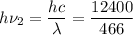
= 26.6 e V
h c = 6.626 × 10⁻³⁴ × 3 × 10⁸ = 12400 e V A°
K + ( 3.4 ) = 26.6 e V
a) energy of free electron
K = 26.6 - 3.4 = 23.2 e V
b) energy of the original photon
h ν₁ - 13.6 = K
h ν₁ = 23.2 + 13.6
= 36.8 e V
energy of the original photon is 36.8 eV