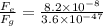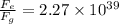Answer:
(a) 8.2 x 10^-8 N
(b) 3.6 x 10^-47 N , 2.27 x 10^39
Step-by-step explanation:
charge of proton, q1 = 1.6 x 10^-19 C
charge of electron, q2 = - 1.6 x 10^-19 C
radius of orbit, r = 0.053 nm = 0.053 x 10^-9 m
mass of electron, me = 9.1 x 10^-31 kg
mass of proton, mp = 1.67 x 10^-27 kg
Gravitational constant, G = 6.67 x 10^-11 Nm^2/kg^2
(a) The electrostatic force between two charges is given by
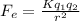
Where, K is the coulombic constant = 9 x 10^9 Nm^2/C^2
By substituting the values
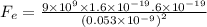
Fe = 8.2 x 10^-8 N
(b) The gravitational force between the electron and proton is given by
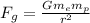
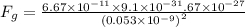
Fg = 3.6 x 10^-47 N