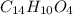Answer : The molecular formula of benzoyl peroxide is
Explanation :
Empirical formula : It is the simplest form of the chemical formula which depicts the whole number of atoms of each element present in the compound.
Molecular formula : it is the chemical formula which depicts the actual number of atoms of each element present in the compound.
For determining the molecular formula, we need to determine the valency which is multiplied by each element to get the molecular formula.
The equation used to calculate the valency is :
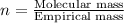
As we are given that the molar mass of compound is, 0.242 kg/mol.
Molecular mass = 0.242 kg/mol = 242 g/mol (1 kg = 1000 g)
The empirical mass of
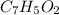 = 7(12) + 5(1) + 2(16) = 121 g/eq
= 7(12) + 5(1) + 2(16) = 121 g/eq
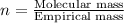
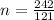
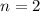
Molecular formula =
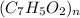 =
=
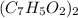 =
=
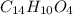
Thus, the molecular formula of benzoyl peroxide is