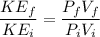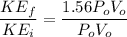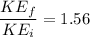Answer:
a)P/Po=0.263
b)γ=1.33 So gas is triatomic
c)
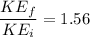
Step-by-step explanation:
a)
initial pressure = Po
Initial volume = Vo
Final volume = 3.8 Vo
Lets take final pressure is P
we know that for free expansion process
PV= Constant
Po x Vo = P x 3.8 Vo
P=0.263 Po
So
P/Po=0.263
b)
Now gas is compressed in adiabatic manner
Final pressure = 1.56 Po
=1.56 Po
We know that for adiabatic process
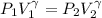
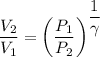
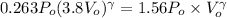
γ=1.33 So gas is triatomic
c)
We know that average kinetic energy given as
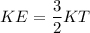
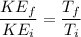 \
\