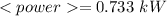Answer:
Step-by-step explanation:
The work W required for the transfer is equal to the energy difference
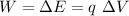
where q is the charge we want to transfer and ΔV is the potential difference.
So, the work needed will be
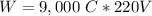
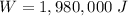
Now, the average power is:
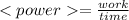
As the Watt is Joule/second, we need the 45 min multiplied by 60:
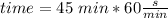
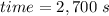
Taking all this together:
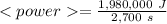
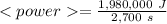
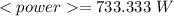
or