Answer:
given,
volume = 133.7 ft³
1 ft³ = 0.0283 m³
133.7 ft³ = 133.7 × 0.0283 = 3.784 m³
mass = 268 kg
1 kg = 2.204 lb
268 kg = 268 × 2.204 = 590.672 lb
weight of the fuel = 268 × 9.8 = 2626.4 N
1 N = 0.2248 lbf
2626.4 N = 2626.4 × 0.2248 = 590.414 lbf
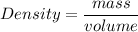
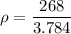
ρ = 70.82 kg/m³
specific volume =
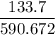
= 0.226 ft³/lbm