Answer:
a) There are
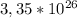 electrons in a liter of water.
electrons in a liter of water.
b) The net charge is -53601707,1 C
Step-by-step explanation:
a) To find out how many electrons are in a liter of water (equivalent to 1000 grams of water), we have to find out how many molecules of water there are and then multiply it by 10 (e- per molecule).
We can find out how many molecules are by finding the number of moles and then multiplying it by Avogadro's number (number of elements per mol):

b) As all electrons have the same charge, in order to find the net charge of those electrons we have to multiply the charge of a single electron by the number of electrons:
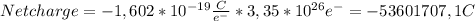
An important clarification is that while the net charge may seem huge, water as a whole is a neutral medium, because there are as many protons as there are electrons, and as they have the same charge, the net charge of water is 0.