Answer:
0.333 L CO2
Step-by-step explanation:
Hello,
I'm attaching the picture with the mathematical procedure for this exercise.
Don't forget to consider that the HCl is in excess because the resulting solution's weight is pretty much similar to the HCl's mass (40 mL*1.14 g/mL=45.6g), so we can say that it is by far in excess, thus, the resulting CO2 is computed based in the initial amount of calcium carbonate as is shown in the picture. One could say that the reaction is likely to be:
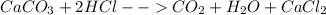
For us to know the stoichiometric relationship.
Best regards.