Answer:
It will take 58 seconds
Explanation:
If you have 1 kg of water in the jar, half of it will be 0.5 kg.
To vaporize half of the water (0.500 kg) the energy supply needed is
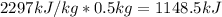
If the stove is supplying 20 kJ/s, the time its needed is
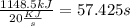
It will last at least 58 seconds to vaporize half of the water.