Answer:
In kJ/kg.K - 1.005 kJ/kg degrees Kalvin.
In J/g.°C - 1.005 J/g °C
In kcal/ kg °C 0.240 kcal/kg °C
In Btu/lbm-°F 0.240 Btu/lbm degree F
Explanation:
given data:
specific heat of air = 1.005 kJ/kg °C
In kJ/kg.K
1.005 kJ./kg °C = 1.005 kJ/kg degrees Kelvin.
In J/g.°C
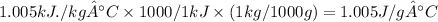
In kcal/ kg °C
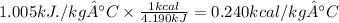
For kJ/kg. °C to Btu/lbm-°F
Need to convert by taking following conversion ,From kJ to Btu, from kg to lbm and from degrees C to F.
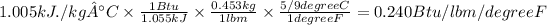
1.005 kJ/kg C = 0.240 Btu/lbm degree F