Answer:
0.8100 g/mL
Step-by-step explanation:
Given,
Volume of the beaker, v = 200.0 mL
Weight of the beaker with oil in it = 292.2 g
Weight of the empty beaker = 130.2 g
Need to find = Density (ρ)
We know that,
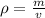
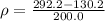
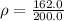

Thus, the density of oil = 0.8100 g/mL