Answer: Option (B) is the correct answer.
Step-by-step explanation:
The given data is as follows.
mass of KCl = 40 g, mass of water = 250.0 g
Hence, number of moles of KCl will be calculated as follows.
No. of moles =

=
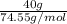
= 0.537 mol
Number of moles of water will be calculated as follows.
No. of moles =

=
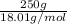
= 13.9 mol
Also, mole fraction of KCl will be calculated as follows.
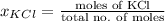
=
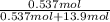
=
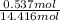
= 0.037
Hence, calculate the vapor pressure of the solution as follows.
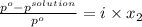
Here, i = 2 because KCl on dissociation produces 2 ions that is,
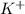 and
and
 .
.
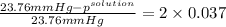
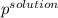 = 22.1 mm Hg
= 22.1 mm Hg
Thus, we can conclude that the vapor pressure of the given solution is 22.1 mm Hg.