Answer:
133 pm
Step-by-step explanation:
Given that the edge length , a of the KCl which forms the FCC lattice = 628 pm
Also,
For the FCC lattice in which the anion-cation contact along the cell edge , the ratio of the radius of the cation to that of anion is 0.731.
Thus,
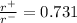 .................1
.................1
Also, the sum of the radius of the cation and the anion in FCC is equal to half of the edge length.
Thus,
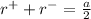 ...................2
...................2
Given that:
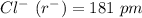
To find,
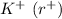
Using 1 and 2 , we get:
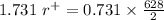
Size of the potassium ion = 133 pm