Answer:
The required mass is 14.741 kg,
Explanation:
Consider the provided information.
Heat of fusion of ice
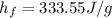
Heat necessary to melt 185 grams of ice at 0 degrees Celsius is:
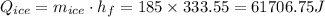
The heat gained by water is:
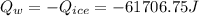
Specific heat capacity of water is
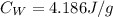 °C
°C
Now use the formula:
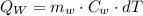
Substituting the respective values in the above formula.
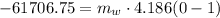
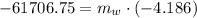
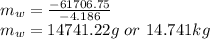
Hence, the required mass is 14.741 kg,