Answer:
d. 2.6 billion years.
Step-by-step explanation:
The half-life of a radioactive element it's the time that its mass decreases by half, or, by 0.50. So, if there is 10 mg of A when the mass became 5 mg, it will have past one half-life. The next half-life will decrease by half the actual mass, so it will give 2.5 mg, which is 0.5x0.5 of the initial mass.
So, the number of half-life (n) can be calculated for:
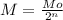
Where M is the residual mass and Mo the inital mass, so:
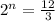
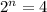
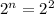
n = 2
So, it has passed 2 half-lives. If one half-life is 1.3 billion years, two must be 2.6 billion years, which is the age of the rock.