Answer:
Balancing is making the number of atoms of each element same on both the sides (reactant and product side).
To find the number of atoms of each element we multiply coefficient and the subscript
For example
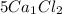 contains
contains
5 × 1 = 5 ,Ca atoms and
5 × 2 = 10, Cl atoms
If there is a bracket in the chemical formula
For example
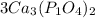 we multiply coefficient × subscript × number outside the bracket.......... to find the number of atoms (Please note: 3 is the coefficient, and if there is no number given then 1 will be the coefficient )
we multiply coefficient × subscript × number outside the bracket.......... to find the number of atoms (Please note: 3 is the coefficient, and if there is no number given then 1 will be the coefficient )
So
3 × 3 = 9 , Ca atoms
3 × 1 × 2 = 6, P atoms
3 × 4 × 2 = 24, O atoms are present.
So
Let us balance the equation given
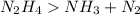
(Unbalanced)
Reactant side element Product
2 N 3
4 H 3
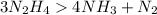
(Balanced)
Reactant side element Product
6 N 6
12 H 12
(Balanced)
N2H4 is Hydrazine which produces NH4 ammonia and nitrogen gas on heating that on decomposition.