Answer : The relationship between the mass of the reactants and the mass of the products is that the mass of the reactants should be equal to the mass of the products.
Explanation :
Law of conservation of matter : It states that matter can neither be created nor be destroyed but it can only be transformed from one form to another form.
The balanced chemical reaction always follow the law of conservation of mass.
Balanced chemical reaction : It is defined as the reaction in which the number of atoms of individual elements present on reactant side must be equal to the product side.
For example :
The given chemical reaction is,
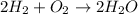
This reaction is a balanced chemical reaction in which number of atoms of hydrogen and oxygen are equal on the both side of the reaction. So, this reaction obey the law of conservation of matter.
Or, we can say that the mass of the reactants is equal to the mass of the products.
In the reaction,
Mass of reactants = Mass of products
Mass of
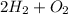 = Mass of
= Mass of
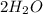
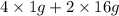 =
=
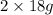
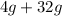 =
=
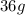
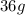 =
=
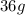
That means, the mass of the reactants should be equal to the mass of the products.