Answer:
a)
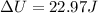
b)
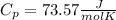
c)
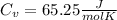
Step-by-step explanation:
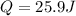
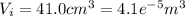
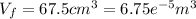
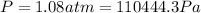
a) The first law of thermodynamics tell us:
ΔU=Q-W
Where ΔU is the variation in internal energy, Q heat and W work done.
Now,
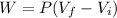 .
.
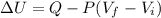 ⇒
⇒
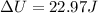
b)
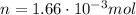
At constant pressure we can write:
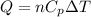 ⇒
⇒
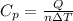
But what's the change in temperature? We can use the Ideal Gas Law:
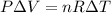 ⇒
⇒
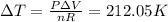
∴
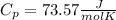
c) Now, we knoe that Cv and Cp are related by Cp-Cv=R.
⇒ Cv=Cp-R ⇒
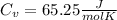 .
.