Answer:
4.15mol/dm³
Step-by-step explanation:
Given:
Mass of LiOH = 20g
Volume of water = 200mL
Unkown:
Molarity of the solution =?
Solution:
Molarity is defined as the number of moles of a given solute in a volume of solution.
Molarity =
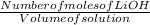
We don't know the number of moles of LiOH,
Number of moles of LiOH =
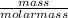
Molar mass of LiOH = 7 + 16 + 1 = 24g/mol
Number of moles of LiOH =
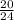 = 0.83mole
= 0.83mole
Note:
1000mL = 1dm³
200mL gives 0.2dm³ of the water.
Molarity =
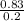 = 4.15mol/dm³
= 4.15mol/dm³