Answer:
CCl₂
Step-by-step explanation:
Let us calculate the mass of carbon present in 100 grams of sample.
The formula to calculate the mass of carbon we can use the following formula.
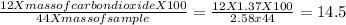
The mass of chlorine in the sample = 100-14.5 = 85.5g
Let us calculate the moles of each element:
moles of carbon =
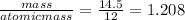
Moles of chlorine =
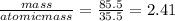
The mole ratio is :
C:Cl = 1.208:2.41 = 1:2
So the empirical formula of the compound must be CCl₂