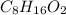Answer:
The empirical formula is =
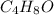
The formula of Valproic acid =
Step-by-step explanation:
Mass of water obtained = 0.166 g
Molar mass of water = 18 g/mol
Moles of
 = 0.166 g /18 g/mol = 0.00922 moles
= 0.166 g /18 g/mol = 0.00922 moles
2 moles of hydrogen atoms are present in 1 mole of water. So,
Moles of H = 2 x 0.00922 = 0.01844 moles
Molar mass of H atom = 1.008 g/mol
Mass of H in molecule = 0.01844 x 1.008 = 0.018588 g
Mass of carbon dioxide obtained = 0.403 g
Molar mass of carbon dioxide = 44.01 g/mol
Moles of
 = 0.403 g /44.01 g/mol = 0.009157 moles
= 0.403 g /44.01 g/mol = 0.009157 moles
1 mole of carbon atoms are present in 1 mole of carbon dioxide. So,
Moles of C = 0.009157 moles
Molar mass of C atom = 12.0107 g/mol
Mass of C in molecule = 0.009157 x 12.0107 = 0.11 g
Given that the Valproic acid only contains hydrogen, oxygen and carbon. So,
Mass of O in the sample = Total mass - Mass of C - Mass of H
Mass of the sample = 0.165 g
Mass of O in sample = 0.165 - 0.11 - 0.018588 = 0.036412 g
Molar mass of O = 15.999 g/mol
Moles of O = 0.036412 / 15.999 = 0.002276 moles
Taking the simplest ratio for H, O and C as:
0.01844 : 0.002276 : 0.009157
= 8 : 1 : 4
The empirical formula is =
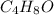
Molecular formulas is the actual number of atoms of each element in the compound while empirical formulas is the simplest or reduced ratio of the elements in the compound.
Thus,
Molecular mass = n × Empirical mass
Where, n is any positive number from 1, 2, 3...
Mass from the Empirical formula = 4×12 + 8×1 + 16= 72 g/mol
Molar mass = 144 g/mol
So,
Molecular mass = n × Empirical mass
144 = n × 72
⇒ n = 2
The formula of Valproic acid =