Step-by-step explanation:
The valency of the element is the measure of the combining power of the element with the other atoms when the element forms compounds or molecules.
Thus, valency has to known to find the how the elements have been combined and how many electrons have been lost, gain or shared.
Given that:
Element A has 3 valence electrons and element B has 2 valence electrons. To find the ionic compound, the valency of the cations and the anions are interchanged and are written in subscripts. Thus,
A B
3 2
Cross multiplying, we get the formula :
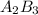
Hence, 3 is the subscript for Element B.