Answer:
Option C, option D and option E is correct
Step-by-step explanation:
Atomic number of O = 8
Electronic configuration of O =
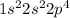
No. of valence electron = 6
Atomic number of S = 16
Electronic configuration of O =
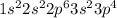
No. of valence electron = 6
Atomic number of Ne = 10
Electronic configuration of Ne =
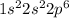
No. of valence electron = 8
No. of electron shell present in any atom is equal to principle quantum no.
So, no. of electron shell in O = 2
no. of electron shell in S = 3
no. of electron shell in Ne = 2
O is present in period 2 and group 16.
S is present in period 3 and group 16.
Ne is present in period 2 and group 16.
So, O and S are present in same group. Elements of same group or family display similar chemical properties.
So, among correct options are:
option C: Oxygen and Neon are in the same period and have the same number of electron shells.
Option D: Oxygen and Sulfur have the same number of valence electrons.
Option E: Oxygen and Sulfur are most similar because they are in the same family and have similar chemical properties.